Pipeline
Eye-Related
Overview
Yong Ding Biopharm’s Eye Health Product Line advances ocular innovation through a strategic partnership with 3D Global Biotech Inc., securing exclusive global distribution rights to FDA-registered ophthalmic solutions. The portfolio includes Exovisse Contact Lens cleared by the U.S. FDA as a Class II medical device (510(k) K213119), offering high comfort, safety, and visual clarity; and Exovisse Artificial Tears cleared by U.S. FDA OTC monograph. Together, these products exemplify Yong Ding Biopharm’s commitment to regulatory excellence and global market readiness in addressing the evolving needs of modern eye care.
Product
Exovisse Artificial Tears
Exovisse Contact Lenses
Product Classification & Regulatory Strategy
US FDA Registration &
Market Approval
European CE Mark
Japan PMDA
Exovisse Artificial Tears
Regulatory Status
In 2025, Exovisse Artificial Tears was successfully registered with the U.S. Food and Drug Administration (FDA) as a OTC monograph.
Product Overview
Our Exovisse artificial tear formulation is designed to relieve symptoms of dry eye disease by restoring moisture, stabilizing the tear film, and improving ocular comfort, providing safe, non-invasive relief for patients with sensitive or irritated eyes.
Millions of people suffer from dry eye symptoms, this drop-based solution delivers hydration and protection to the ocular surface using a gentle formulation ideal for daily use. By improving tear film stability and moisture retention, the drops reduce discomfort caused by dry eye syndrome, ocular irritation, and environmental stressors.
Next Steps
Ongoing development efforts aim to expand the Exovisse platform. R&D initiatives are focused on integrating comfort-enhancing biomaterials and potentially combining with therapeutic agents or adjunctive lens care systems for future next-generation ophthalmic solutions.

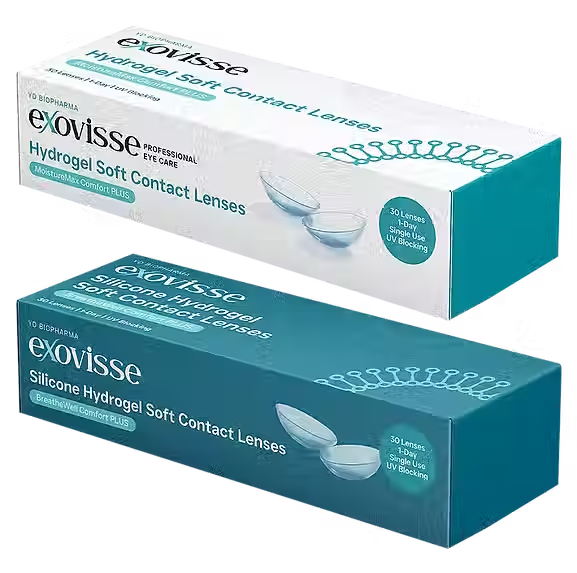
Exovisse Contact Lens
Regulatory Status
In 2025, Exovisse Contact Lens, a soft disposable contact lens developed by 3D Global Biotech Inc., received U.S. FDA 510(k) clearance (K213119) as a Class II Medical Device under the classification Lenses, Soft Contact, Daily Wear (21 CFR 886.5925). The product is registered with the FDA under Product Codes LPL and MVN, confirming its regulatory compliance for ophthalmic use in the United States.
Product Overview
Exovisse Contact Lens is a soft contact lens designed for daily wear, developed to provide optimal comfort, corneal oxygenation, and long-lasting hydration throughout the day. Engineered with advanced biocompatible polymers, ExoLens offers high optical clarity and surface stability, making it suitable for routine visual correction and all-day ocular health support.
Its FDA Class II designation assures rigorous quality, safety, and performance testing, including biocompatibility, lens material characteristics, and sterility, in compliance with the U.S. FDA’s medical device regulations.
Next Steps
Ongoing development efforts aim to expand the Exovisse platform. R&D initiatives are focused on integrating comfort-enhancing biomaterials and potentially combining with therapeutic agents or adjunctive lens care systems for future next-generation ophthalmic solutions.